But its these polyatomic ions that get me confused. Thanks a lot for your help! But I remember the easiest way to figure out if a compound is Ionic or covalent is to take the difference of the For a compound that is composed of just nonmetals, CO2 for example, the bonds are usually covalent.What Is the Difference Between Ionic vs Covalent Bonds? Ionic compounds have a metal, and covalent compounds don't. Learn why. Here's the first thing to know about ionic vs covalent substances. Covalent compounds are held together by covalent bonds.To tell if CO2 (Carbon dioxide) is ionic or covalent (also called molecular) we look at the Periodic Table that and see that C is a non-metal and O is a non...Covalent bond. CO2. We get answers from Resources: answers.yahoo.com answers.com google.com youtube.com pubchem.ncbi.nlm.nih.gov reference.com www.quora.com Is OCl2 ionic or Covalent bond ?Mitigation of anthropogenic CO2 has become a global challenge to prevent the negative consequences associated with high atmospheric CO2 concentration. Scheme 5. NOHMs: (A) overview of their structure with either ionic or covalent bonding between the canopy and the functionalized core; (B)...
Ionic vs Covalent | Easy Hard Science
Ions are formed when an atom loses or gains an electron. These types of bonds are commonly One atom of carbon (C) combines with two atoms of oxygen (O) to form a double covalent bond in carbon dioxide (CO2). However, it is not an ionic or covalent bond but is a particular type of dipole-dipole...II: Exercises. Part A: Covalent or Ionic Compound? Characteristics of Covalent and Ionic Compounds. Consider Table 1 when answering the following questions. dinitrogen tetroxide. CO2. carbon dioxide. PCl3. phosphorus trichloride. CO. carbon monoxide.The ions then attract each other through electrostatic forces of attraction as they are oppositely charged. Covalent bonding occurs when The outer shell electrons of metals are delocalised (free to move around) and so a metallic structure is a regular arrangment of +ve charged ions with negative...Chemistry · 1 decade ago. is CO2 an ionic or covalent compound? If both are non-metallic, then it's covalent bonding. If there's one of each it is ionic. As both carbon and oxygen are non-metals, the bonds are covalent.

Is CO2 (Carbon dioxide) Ionic or Covalent/Molecular? - YouTube
Ionic and covalent bonds hold molecules together. The only pure covalent bonds occur between identical atoms. Usually, there is some polarity (polar covalent bond) in which the electrons are shared, but spend more time with one atom than the other.CO2 is Covalent a covalent bond is formed between two non-metals in this case carbon and oxygen Is carbon (iv)oxide a covalent or ionic bond? Chemical bond A chemical bond is a lasting attraction between atoms, ions or molecules that enables the formation of chemical compounds.Ions exert electrostatic force on each other, which forms ionic bonds. The hydrogen and oxygen Covalent bonding generally happens between nonmetals. Covalent bonding is the type of bond that Similarly, in carbon dioxide (CO2), two double bonds are formed between the carbon and each of the...Start studying Chapter 2: Ionic & Covalent Compounds. Learn vocabulary, terms and more with flashcards, games and other study tools. ions in solution that were ionic compounds, which got dissociated/dissolved in water, surrounded by - CO is carbon monoxide, an exception to 1st rule.Ionic or covalent? I've gotten conflicted answers when I googled this question online. Ionic Bonds are stronger than covalent bonds because the electronegativity difference between the two elements is much greater than that of two elements in...
Yahoo Answers has shut down as of May 4, 2021. Yahoo Answers used to be once a key a part of Yahoo's services, however it has declined in recognition over time as the desires of our contributors have changed. We made up our minds to shift our assets away from Yahoo Answers to focus on merchandise that better serve our individuals and deliver on Yahoo's promise of offering premium trusted content.
As of May 4, 2021 you'll no longer access the web page, but you'll still request a obtain of your Yahoo Answers data until June 30, 2021. To assist you with this transition we've got compiled an inventory of questions that can arise right through this process.
Will this have an effect on my Yahoo Account or other Yahoo products and services?No, these adjustments are particular to Yahoo Answers. They would possibly not affect your Yahoo Account or another Yahoo services.
Where will have to I am going when I have questions at some point?Yahoo Search can be utilized to seek out solutions and information from the web. Our Yahoo COVID page supplies info and assets concerning the Coronavirus pandemic.
Can I obtain my Yahoo Answers content? What content material is to be had to me?Your Yahoo Answers knowledge download will go back all user-generated content, including your questions, answers and photographs. You won't be able to download other customers' content material, questions, or solutions.
Do I have to download my content?No, the content material download is non-compulsory. However, if you select to obtain your content material, you might have to do so earlier than June 30, 2021.
When will I get my Yahoo Answers content?Our workforce works as fast as conceivable to make data to be had, but it will probably take as much as 30 days to receive your content download.
I downloaded my Yahoo Answers content material, how do I view it?Your content material will probably be formatted in JSON (JavaScript Object Notation) and could be exhausting to appear thru at a glance. We have assets on viewing and managing your account knowledge that can help you understand your information download.
How can I proportion my feedback/comments about this transformation?Send any feedback or comments you may have regarding this determination to yahoo_answers_sunset@verizonmedia.com. Thanks for taking the time to proportion your thoughts with us.
PPT - How many valence electrons does magnesium have ...

PPT - How many valence electrons does magnesium have ...

Solved: Classify The Solid State Of The Following Substanc ...

Types of Covalent Bonds: Polar and Nonpolar | manoa.hawaii ...

MIT Researchers Figure Out How to Turn Carbon Emissions ...

PPT - How many valence electrons does magnesium have ...

PPT - How many valence electrons does magnesium have ...

PPT - How many valence electrons does magnesium have ...

PPT - How many valence electrons does magnesium have ...
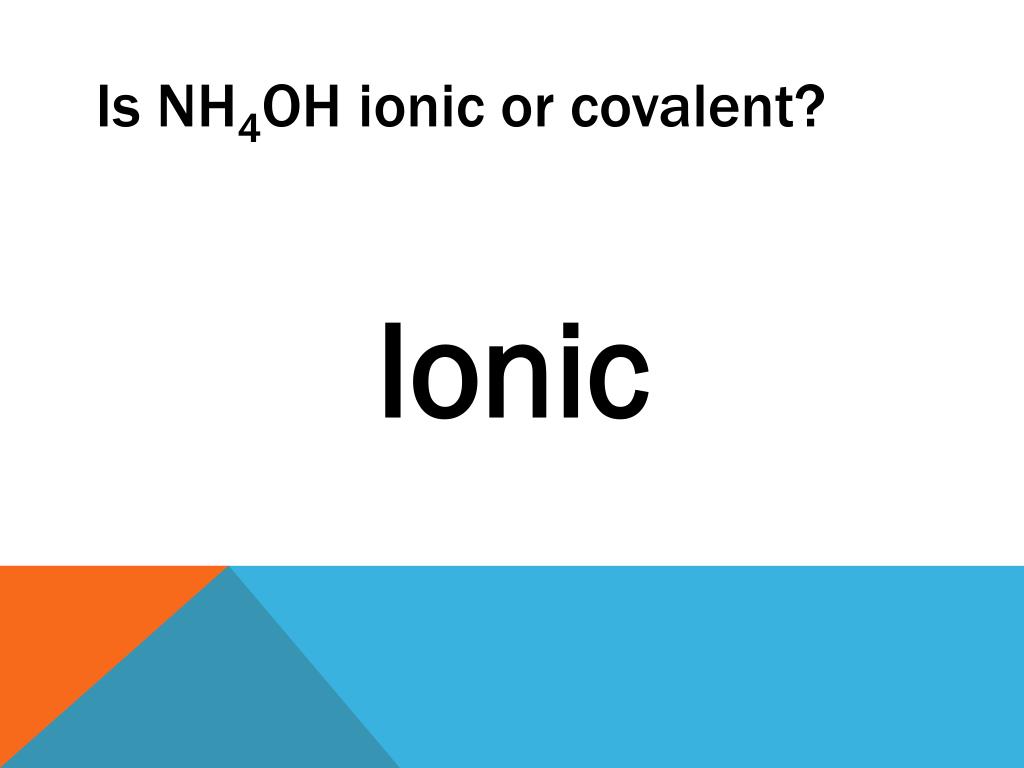
What kind of bond type is ammonia? - Quora
MIT Researchers Figure Out How to Turn Carbon Emissions ...

ما هي الرابطة التساهمية ؟ - أنا أصدق العلم

Examples of Covalent Bonds | Uses and Diagrams | Chemistry ...

What is the type of bonding in carbon dioxide? - Quora
What are some examples of ionic, covalent and metallic ...
PPT - How many valence electrons does magnesium have ...

PPT - How many valence electrons does magnesium have ...

PPT - How many valence electrons does magnesium have ...

PPT - How many valence electrons does magnesium have ...

Strength of Polar Covalent Bond

Learning about Covalent Bonding - Chemistry Revision - Ask ...







No comments:
Post a Comment