Isotope. Isotopes are different forms of the same element that are different from each other The difference in the number of neutrons between the various isotopes of an element means that the The stable isotopes have nuclei that do not decay to other isotopes on geologic timescales, but may...Isotopes are different forms of an element whit the same number of protons but a different number of neutrons with practically the same chemical properties. Usually, what makes an atom unstable is the large nucleus. If a core becomes large enough from the number of neutrons, it will be inconsistent.Isotopes of an element have nuclei with a different number of protons, but the same number of electrons. Isotope: The atoms of element having same atomic number but different mass number are called isotope. Hydrogen has three isotopes called as.Different isotopes of an element have different numbers of neutrons in their nucleus. For example, the stable isotope carbon-12, the most common type of carbon in the human body, has Z=6 and N=6, while carbon-14, the radioactive isotope used in carbon dating, has Z=6 and N=8. There are less...Isotopes are atoms of the same element that vary in their respective numbers of neutrons, the neutral particle in the nucleus of an atom. Carbon-14, a radioactive isotope of carbon with 6 protons and 8 neutrons, is used to date fossils because its rate of radioactive decay is known.
What Is an Isotope? Definition, Types and Examples
Question.2 The elements of the third period of the Periodic Table are given below: (a) Which atom is Chemical properties do not depend upon atomic mass. (ii) Isotopes have different atomic mass but How do the following properties of M and N vary? Sizes of their atoms. Their metallic characters.All elements above lead in the periodic table, like Tc and Pm, are decaying away. Those atoms above lead, however, generally have at least one long In other words, chemically all isotopes of the same element make the same chemical connections with other elements and are thus macroscopically the...The question arises of how atomic nuclei that contain multiple protons can exist - orbited by multiple electrons Increasing the number of protons leads to different chemical elements. Some unstable isotopes (some that decay in a fraction of a second, or after thousands of years) were formed when...4.8: Isotopes - When the Number of Neutrons Varies - Chemistry 6 Feb 2021 Different isotopes of an element generally have the same physical Carbon atoms with 7 neutrons have Atomic structure refers to the structure of an atom comprising a nucleus (centre) in which the protons...

How do the nuclei of different isotopes of an element vary?
Isotopes and atomic masses. For many of the chemical elements there are several known The number of electrons also may vary, but only if the atom ionizes, and in any case the relative As a consequence the molar masses of the different isotopes of an element are significantly different.Predicting the stability of an isotope introductory tutorial with worked examples. It is believed that the stability of the nucleus of an isotope is determined by the ratio of neutrons to protons. The table below lists some stable and unstable isotopes (radioisotopes) of a number of different elements...Isotopes differ in the number of neutrons, fundamental, massive, neutral nuclear particles, their atoms contain. And thus all isotopes of the same element necessarily contain "THE SAME NUMBER OF Most elements have several different isotopes, and their weighted average gives rise to the...Isotopes are different nuclear configurations of the same element which vary in the number of neutrons present between each other. These elements are still technically the same element, because elements are classified according to the number of protons. Isotopes do not interact any differently...Since an element's isotopes have slightly different mass numbers, the atomic mass is calculated by obtaining the mean of the mass numbers for isotope: Any of two or more forms of an element where the atoms have the same number of protons, but a different number of neutrons within their nuclei.
Chemical homes are all the same. The handiest physical houses I'm conscious of that can exchange between isotopes are the mass of the atom, the density of the atom, and the half life of the element. Since the mass of the element is different between isotopes, underneath a selected influence of kinetic energy, say a applied electric box, thus the pace maximum reached below that affect will vary--lighter components shifting sooner.
Isotopes are often used in analytical chemistry for radiolabeling as a result of the bodily houses are an identical between isotopes, making them superb for use as internal standards.
Also, I've noticed that the triple segment diagrams of some isotopes differ relatively quantitatively and qualitatively.
PPT - Basic Chemistry PowerPoint Presentation, free ...

Mr. Gortney's 8th Grade Science Class / Class Notes

chemistry chap 4 study - Atom The smallest particle of an ...

Atomic number, mass number, and isotopes | Chemistry ...

Physical science reading and study workbook chapter 4.2 ...

How Nuclear Weapons Work

Power Notes - Atomic Structure 2013
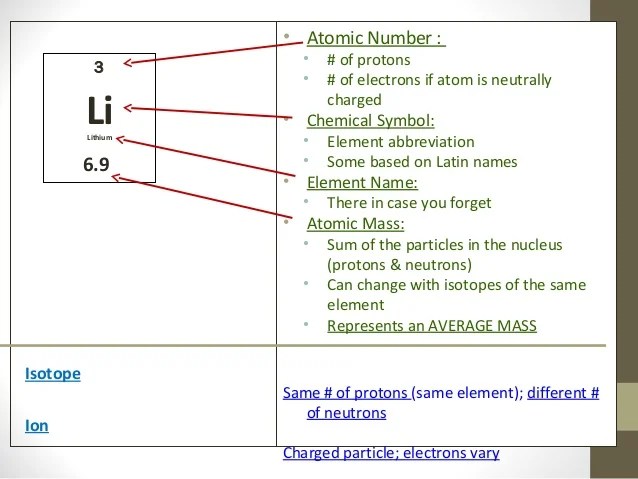
PPT - Atomic Structure and the Periodic Table PowerPoint ...
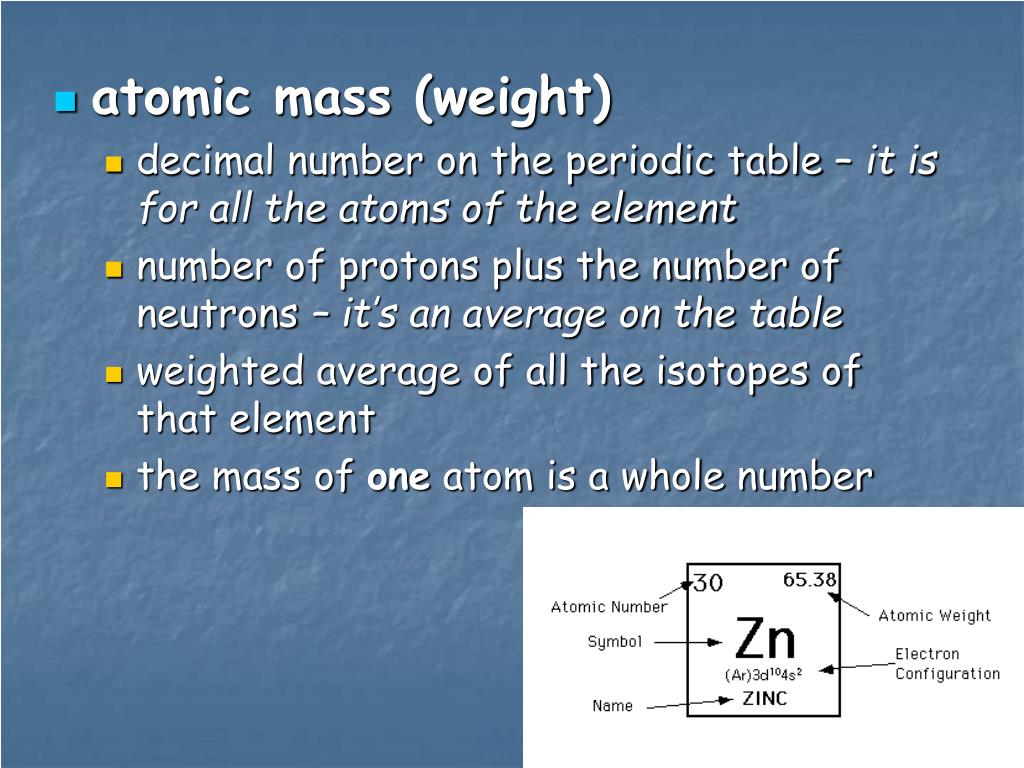
Atomic Structure & The Changing Models of Atom

PPT - An atom is the smallest particle of an element that ...

Radioisotopes Applications in Nuclear Medicine and Nuclear ...

giant presentation on model of an atom
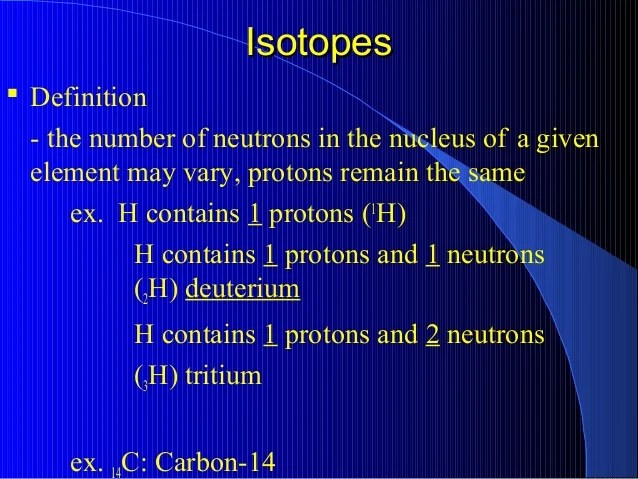
Radioactive Dating: Questions Answered | Answers in Genesis

giant presentation on model of an atom

I might have found the origin of Superman's name : DCcomics

PPT - Introduction to atoms and molecules PowerPoint ...

Isotopes2
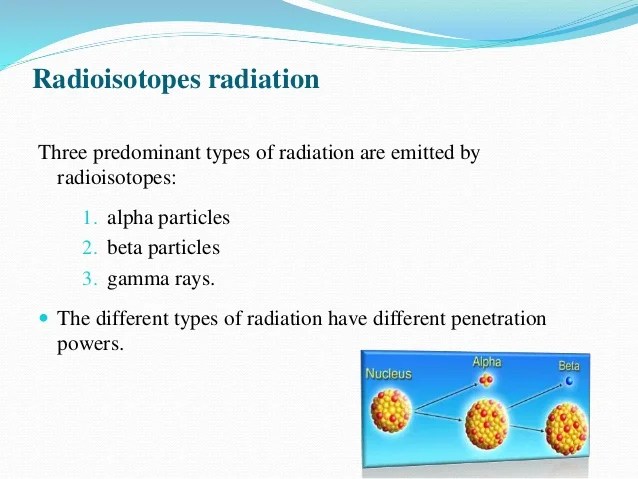
Chapter 2 - Environmental Systems Chapter 2 Learning ...

The Nucleus: The Center of an Atom - dummies

MakeTheBrainHappy: Isotope Notation

How many neutrons can you fit in an atomic nucleus? More ...







No comments:
Post a Comment