molecular weight of Pb (C2H3O2)2 or mol This compound is also known as Lead (II) Acetate. The SI base unit for amount of substance is the mole. 1 grams Pb (C2H3O2)2 is equal to 0.0030741984857482 mole.Pb + Zn(C2H3O2)2 -> Zn + Pb(C2H3O2)2. 2AgNO3 + Ni-> Ni(NO3)2 + 2Ag. Which is a characteristic of a decomposition reaction? Substance P replaces X in the compound XY. Substance XYZ breaks down into X, Y, and Z. Substance Z burns in the presence of oxygen to form ZO.Balance the reaction of Pb + Zn(C2H3O2)2 = Zn + Pb(C2H3O2)2 using this chemical equation balancer!Pb (C2H3O2)2 molecular weight Molar mass of Pb (C2H3O2)2 = 325.28804 g/mol This compound is also known as Lead (II) Acetate. Convert grams Pb (C2H3O2)2 to moles or moles Pb (C2H3O2)2 to gramsThe reaction is an example of a metathesis reaction, which involves the exchange of ions between the Pb(NO3)2 and KI. The Pb+2 ends up going after the I- resulting in the formation of PbI2, and the K+ ends up combining with the NO3- forming KNO3. NO3- All nitrates are soluble.
Types of reactions Flashcards | Quizlet
There are three main steps for writing the net ionic equation for Pb(C2H3O2)2 + KI = KC2H3O2 + PbI2 (Lead acetate + Potassium iodide). First, we balance theProblem: Write the chemical formula for the cation present in the aqueous solution of Pb(C2H3O2)2. FREE Expert Solution. Pb(C 2 H 3 O 2) 2 → salt of acetate (CH 3 COO-) → always soluble. Dissolution of Pb(C 2 H 3 O 2) 2 will appear as: 89% (57 ratings) Problem Details.HI(aq)+Pb(C2H3O2)2(aq)→? Predict the products of each of these reactions and write balanced molecular equations for each. If no reaction occurs, write NOREACTION.With water it forms the trihydrate, Pb(CH3COO)2·3H2O, a colourless or white efflorescentmonocliniccrystallinesubstance. The substance is used as a reagent to make other lead compounds and as a fixative for some dyes. In low concentrations, it is the principal active ingredient in progressive types of hair colouringdyes.
Pb + Zn(C2H3O2)2 = Zn + Pb(C2H3O2)2 - Chemical Equation
Pb(C2H3O2)2 + 2 NaCl ----> 2 Na(C2H3O2) + PbCl2. 0 0. Still have questions? Get answers by asking now. Ask question + 100. Join Yahoo Answers and get 100 points today. Join. Trending questions. Trending questions. Why don't ships rust? 10 answers. Who is the best Mosquito Repellent chemical Manufacturer?Lead Acetate - Pb (C2H3O2)2 What is Lead Acetate? Lead Acetate is also known as lead (II) acetate is a white crystalline chemical compound with the formula Pb (C 2 H 3 O 2) 2. Lead Acetate is poisonous in nature.Molar Mass: 325.288. Example Reactions: • 2 KCl + Pb(C2H3O2)2 = PbCl2 + 2 KC2H3O2Answer: pb (c2h3o2)2 (Lead (II) acetate) is Soluble in water What is Soluble and Insoluble ?I'm studying for a chemistry test that I have tomorrow morning (wish me luck), and need a little bit of help on a practice problem. The solutions are mixed together to form a single solution. One contains 0.2 mol (C2H3O2)2, , the second contains 0.1 mol Na2S, and the third contains 0.1 mol CaCl2. a)Write the net ionic equation for the precipitation reaction or reactions that occur.
Molar mass of Pb(C2H3O2)2 = 325.28804 g/mol
This compound is sometimes called Lead(II) Acetate.
Convert grams Pb(C2H3O2)2 to moles or moles Pb(C2H3O2)2 to grams
Molecular weight calculation:207.2 + (12.0107*2 + 1.00794*3 + 15.9994*2)*2
Element Symbol Atomic Mass # of Atoms Mass %Lead Pb 207.2 1 63.697%Hydrogen H 1.00794 6 1.859%Carbon C 12.0107 4 14.769%Oxygen O 15.9994 4 19.674%Note that all formulas are case-sensitive. Did you imply to seek out the molecular weight of the sort of similar formulation?PB(C2H3O2)2Pb(C2H3O2)2
In chemistry, the components weight is a quantity computed by means of multiplying the atomic weight (in atomic mass devices) of each and every element in a chemical components through the choice of atoms of that element present within the formula, then including all of these merchandise together.
The atomic weights used in this website come from NIST, the National Institute of Standards and Technology. We use the most typical isotopes. This is how to calculate molar mass (reasonable molecular weight), which is in response to isotropically weighted averages. This is not the similar as molecular mass, which is the mass of a unmarried molecule of well-defined isotopes. For bulk stoichiometric calculations, we're most often figuring out molar mass, which can also be referred to as same old atomic weight or reasonable atomic mass.
If the formulation utilized in calculating molar mass is the molecular system, the formula weight computed is the molecular weight. The proportion by weight of any atom or workforce of atoms in a compound can be computed through dividing the full weight of the atom (or workforce of atoms) in the method by way of the formulation weight and multiplying by way of 100.
Finding molar mass starts with units of grams in keeping with mole (g/mol). When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance. The components weight is solely the burden in atomic mass units of all of the atoms in a given system.
Formula weights are especially helpful in figuring out the relative weights of reagents and products in a chemical response. These relative weights computed from the chemical equation are also known as equation weights.
A not unusual request on this web site is to convert grams to moles. To entire this calculation, you need to know what substance you are attempting to convert. The reason why is that the molar mass of the substance impacts the conversion. This site explains how you can in finding molar mass.
Using the chemical formula of the compound and the periodic table of parts, we will upload up the atomic weights and calculate molecular weight of the substance.
What Is The Net Ionic Equation For Reaction Between Pb ...

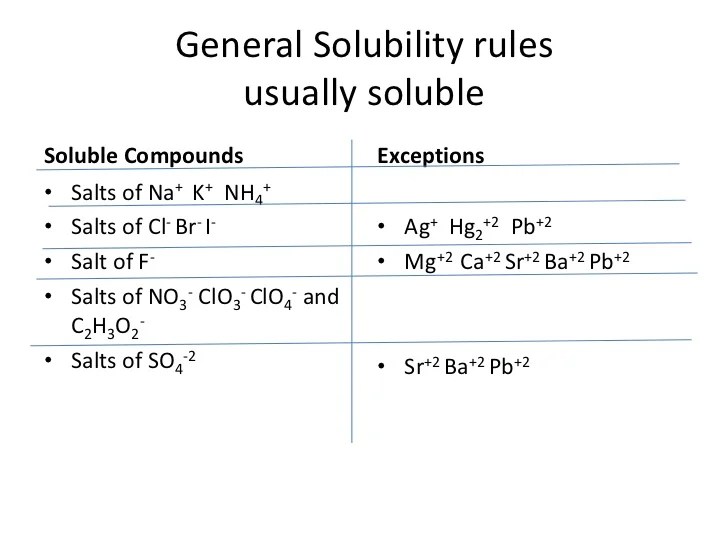
Solubility rules usually soluble

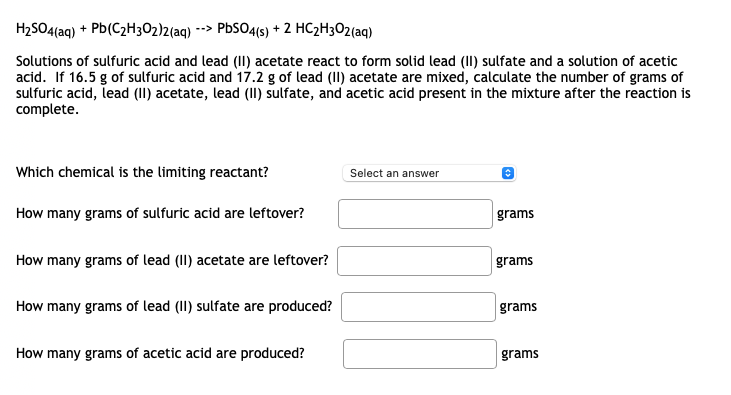
Answered: H2SO4(aq) + Pb(C2H3O2)2(aq) -->… | bartleby
ACETATO DE PLOMO Pb(CH3COO)2.3H2O

Chemistry Archive | October 24, 2016 | Chegg.com
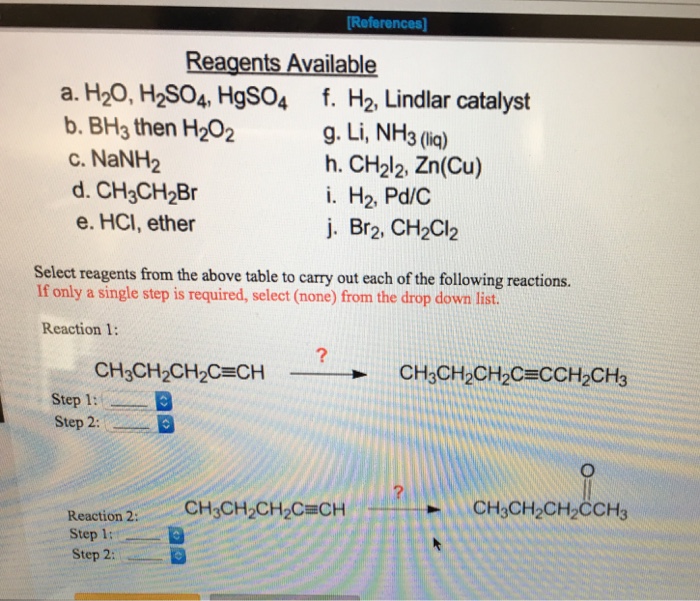
PPT - Reaction Stoichiometry: Mole Method Calculations ...

5) Is a chemical reaction expected to occur if solutions ...

Solved: 1. Lead(II) Bromide, PbBr2, Is Slightly Soluble In ...

Write The Balanced Net Ionic Equation For Dissociation Of ...

Solved: 33. Balance The Following Equations, And Then Writ ...

Solved: 9. 0.1/0.1 Points| Previous Answers 1/4 Submission ...

Solved: 3 Which One Of The Following Compounds Will Be Ins ...

Solved: 1.The Amount Of I3-(aq) In A Solution Can Be Deter ...

pb - syjennifernb12のブログ
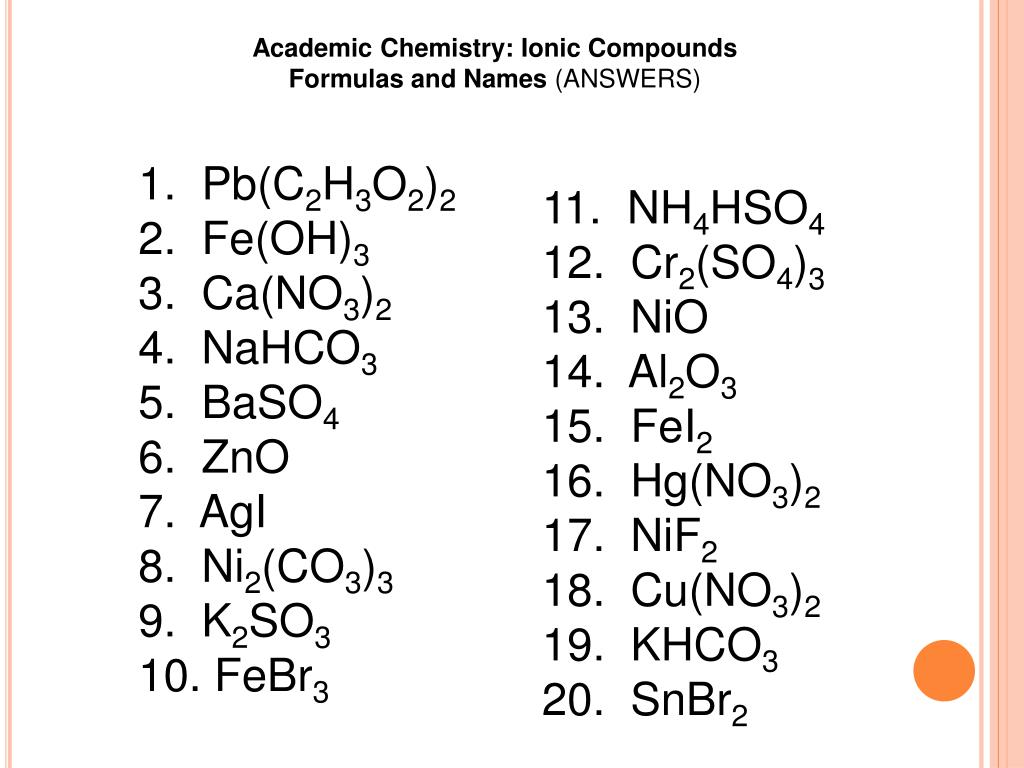
PPT - 1. Pb (C 2 H 3 O 2 ) 2 2. Fe(OH) 3 3. Ca (NO 3 ) 2 4 ...
लेड एसीटेट (Lead Acetate Chemical Formula) का रासायनिक ...

Lead Acetate trihydrate, pure - min 99% - Pb - Catalog ...

PPT - Unit # 4: Aqueous Reactions and Solution ...
Chemical Reactions and Stoichiometry A. Writing and ...

Exporter of Chemical Supplies from Mumbai by A. B. ENTERPRISES

Chemistry - Chp 11 - Chemical Reactions - Notes







No comments:
Post a Comment