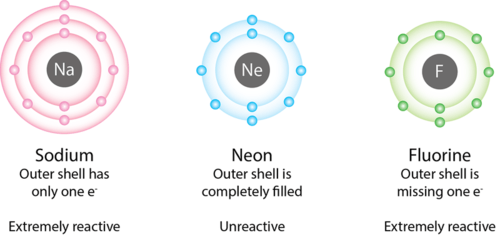
Neon has eight valence electrons, a full octet that makes neon quite stable (Noble gas). This can be confirmed via it's electronic configuration by looking at the highest energy level which is 2 in this case.The number of electrons in each element's electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. In the periodic table, the elements are listed in order of increasing atomic number Z. Electron configuration of Neon is [He] 2s2 2p6. Possible oxidation states are 0.Solved: How many valence electrons does neon have? By signing up, you'll get thousands of step-by-step solutions to your homework questions. You...It is a noble gas, so it has a full octet, with eight electron dots around the outside of the symbol, Ne, though I can't draw it, you can picture it as two dots on each side of the symbol. As with all noble gases the electron configuration has a full octet of s2p6. IN the case of Neon it would be 2s22p6Neon, Z = 10, has eight valence electrons. This closed shell configuration makes neon supremely difficult to oxidize, and difficult to reduce. The inertness, the lack of reactivity, of this Noble Gas, is a function of its electronic configuration.
Neon - Periodic Table and Atomic Properties
A valence electron is an outer shell electron and may participate in the formation of a chemical bond. Ok but how many valence electrons does an atom of Neon have? In the case of Neon the valence electrons is 0. Now let's check the facts about Neon...Those 8 electrons are the outer electrons, and therefore are called valence electrons. The goal for atoms is to gain 8 electrons in the outer shell (valence electrons) and therefore neon is "happy" (does not need to lose or gain any).How Many Valence Electrons Does Neon Have?||Number of Valence Electrons in Neon?||How many valence electrons are in a neutral atom of neon?Read Blog post:htt...Neon Valence Electrons Neon Valency Ne With Dot Diagram . Peoi Introductory Chemistry . Electron Dot Structure . Drawing Lewis Dot Diagrams Set 1 Step By Step Worksheet Worksheet Template Printable Worksheets Chemistry Education . Chapter 8 Chemical Bonds Che 105 110 Introduction To Chemistry Textbook Libguides At Hostos Community College Library

How many valence electrons does neon have? | Study.com
Neon has 8 valence electrons, which gives it a full shell so it cannot interact with outher elements. This is why it is called a noble gas.In fact, the number of valence electrons goes up by one for each step across a period until the last element is reached. Neon, with its configuration ending in s2p6, has eight valence electrons.Periodic Table of Elements with Valence Electrons Trends. In the below periodic table you can see the trend of Valence Electrons. For facts, physical properties, chemical properties, structure and atomic properties of the specific element, click on the element symbol in the below periodic table.Neon atoms have 10 electrons and the electronic shell structure is [2, 8] with Atomic Term Symbol (Quantum Numbers) 1 S 0. Atomic Number can become lengthy and so an abbreviated notation is used.This is important as it is the Valence electrons 2s2 2p6, electrons in the outermost shell that determine the chemical properties of the element.We know that the atomic number of neon is 10.So neon has 10 protons and 10 electrons as the charge of electrons and protons are equal but opposite in nature.The charge of proton is +1 and the charge of electron is -1. Step-3: Now write the electron configuration of neon. Ne (10)=1s²2s²2p⁶
Neon is a p-Block component and belongs to Noble gas or Inert Gas group. Atomic collection of Neon is 10 and Electronic configuration of Neon is:\(1s^22s^22p^6\) It has utterly stuffed valence shell i.e. outermost shell comprises 8 electrons and Rule of Octet is satisfied and so it's solid. So, the valency of Neon is 0.
Ionic Bonds and Ionic Compounds | CK-12 Foundation
PPT - Atomic Structure PowerPoint Presentation - ID:5055482

Lecture 1 - Composition of the atmosphere

PPT - Chapter 6.2 Covalent Bonding and Molecular Compounds ...

Energy Of An Electron | Valence Electrons | whatwho.com

Valence Electron: Definition, Configuration & Example ...

Neon project - Screen 7 on FlowVella - Presentation ...

Reading: Electrons | Biology I

1-2-2. Atoms, Isotopes, Ions, and Molecules: The Building ...

Beryllium diagram | Atom diagram, Electron configuration ...

Elements and Atoms: The Building Blocks of Matter ...

What Is the Number of Valence Electrons in the Outer Shell ...

How To Find The Electron Configuration For Thorium ...

How many unpaired electrons are there in t... | Clutch Prep
Ne Neon - Element Information, Facts, Properties, Trends ...

{Wiring Diagram} Electron Dot Diagram Of Neon

Scientific Explorer: Atoms Part 4D: Atoms and Chemistry ...

Periodic Table Family Names Valence Electrons - Periodic ...

File:Electron shell 010 Neon - no label.svg - Wikimedia ...

Valence Electrons | CK-12 Foundation

[5 Steps] Electron Configuration for or of Neon in Just 5 ...
![[5 Steps] Electron Configuration for or of Neon in Just 5 ... [5 Steps] Electron Configuration for or of Neon in Just 5 ...](https://i0.wp.com/4.bp.blogspot.com/-jMs9FCVMeSw/XD4FtZiSVlI/AAAAAAAAYZk/5zKPuhhk0l486HlF9jaCPN9uUxhdCxIigCEwYBhgL/s1600/20190115_220744.jpg)






No comments:
Post a Comment